Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T02703 | ||||
| Target Name | Nitric-oxide synthase inducible | ||||
| Synonyms | HEP-NOS; Hepatocyte NOS; INOS; Inducible NOS; Inducible nitric oxide synthase; NOS, type II; NOS2 | ||||
| Target Type | Clinical Trial | ||||
| Gene Name | NOS2 | ||||
| Biochemical Class | Oxidoreductases acting on paired donors | ||||
| UniProt ID | NOS2_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Osteoarthritis | ||||
| Example drug | SD-6010 | Phase 3 | [523634], [1572592] | ||
| Tissue | Synovial tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.27 Z-score: -0.88 P-value: 4.24E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
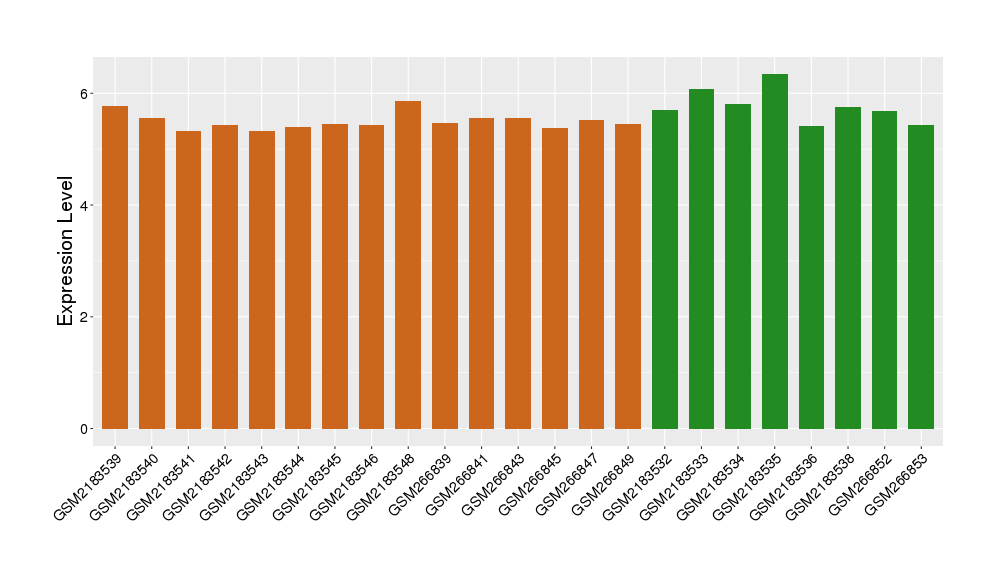
|
|||||
| Disease | Asthma | ||||
| Example drug | GW274150 | Phase 2 | [521884], [1572592] | ||
| Tissue | Nasal and bronchial airway | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.74 Z-score: 0.70 P-value: 5.49E-08 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Coronary artery disease | ||||
| Example drug | LT-1951 | Phase 1/2 | [521773], [1572592] | ||
| Tissue | Peripheral blood | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.02 Z-score: -0.09 P-value: 9.15E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
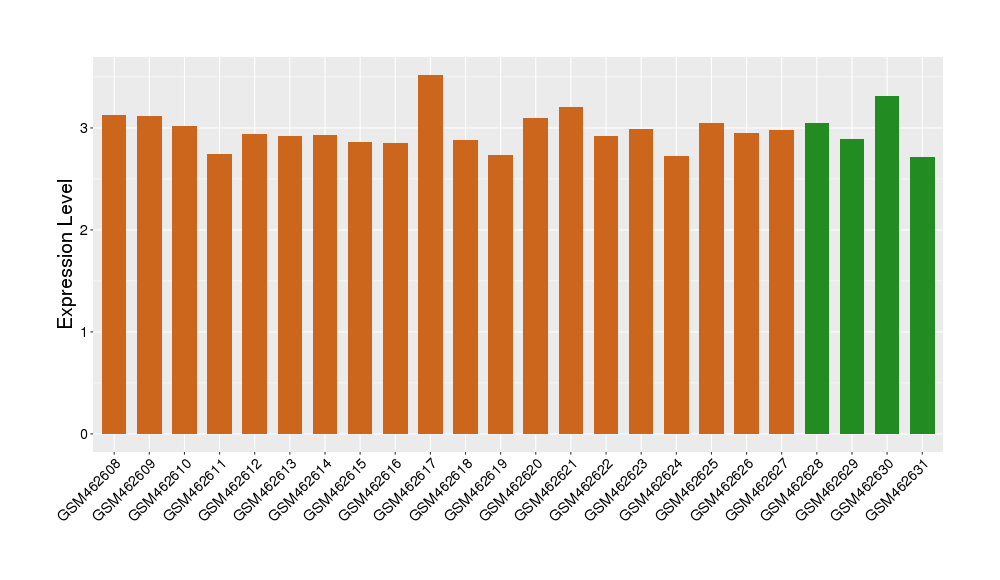
|
|||||
| Disease | Septic shock | ||||
| Example drug | ONO-1714 | Discontinued in Phase 1 | [547187], [1572592] | ||
| Tissue | Whole blood | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.07 Z-score: -0.26 P-value: 6.14E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
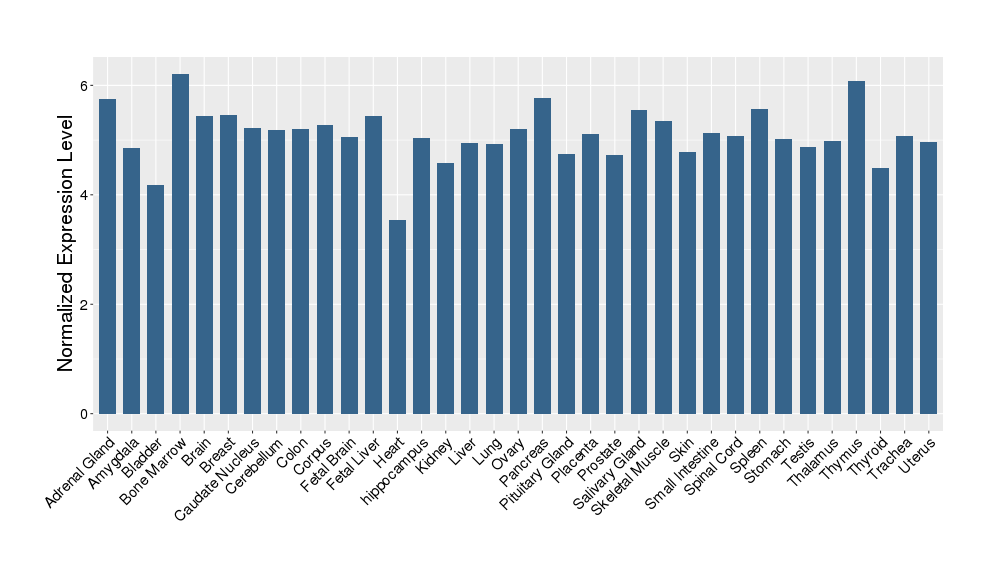
|
|||||
| Reference | |||||
| Ref 547187 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013758) | ||||
| Ref 521773 | ClinicalTrials.gov (NCT00264706) PolyArginine Treated vEiN grafTs (PATENT). U.S. National Institutes of Health. | ||||
| Ref 521884 | ClinicalTrials.gov (NCT00370435) Study Of 90mg Of GW274150 In Subjects Over 50 Years, Who Have Rheumatoid Arthritis (RA). U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Tang and Dr. Zhang.
